Avoid These Key Account Management Mistakes in Clinical Research
Key account management (KAM) in clinical research involves strategically managing high-value client relationships—such as pharmaceutical companies, biotech firms, and research institutions—to drive long-term growth and collaboration. 🏥 In this highly regulated, project-based industry, KAM is crucial for securing repeat business, fostering trust, and navigating complex compliance requirements. Avoiding common mistakes is essential; errors can lead to client dissatisfaction, lost revenue, and reputational damage. For instance, neglecting personalized communication or failing to align with client goals may result in contract terminations.
Practical tips to get started:
- Identify key accounts based on revenue potential and strategic fit.
- Develop customized service plans for each account.
- Use checklists for regular client check-ins and compliance audits.
By prioritizing these steps, you can strengthen relationships and safeguard your business's success in the competitive clinical research landscape. ✅
Mistake 1: Inadequate Client Needs Assessment
Inadequate client needs assessment is a critical mistake in clinical research key account management. 🔍 Many organizations fail to conduct thorough initial assessments and neglect ongoing evaluations, missing evolving client priorities and regulatory changes. This oversight leads to misaligned services that don't address specific client goals or pain points, potentially derailing trials and harming profitability.
Practical steps to avoid this:
- Conduct comprehensive initial assessments using structured interviews and data analysis.
- Implement quarterly review meetings to track evolving priorities.
- Create client-specific dashboards monitoring regulatory compliance and project milestones.
- Develop customized service alignment checklists for each account.
- Establish regular feedback loops with client stakeholders.
Key tips: Always validate assumptions with client data, maintain regulatory change alerts, and ensure service offerings directly address documented pain points. This proactive approach prevents costly misalignments and strengthens long-term partnerships. 📈
Mistake 2: Poor Communication and Engagement Strategies
Poor communication and engagement strategies can derail key account management in clinical research. 📞 Inconsistent or infrequent communication—such as sporadic updates or delayed responses—erodes trust and leaves accounts feeling neglected. Additionally, lack of personalized engagement, like generic reports, fails to demonstrate value and align with trial-specific goals.
To avoid this, implement these actionable strategies:
- Schedule bi-weekly check-ins and send monthly progress summaries.
- Use automated reminders to ensure no follow-ups are missed.
- Tailor interactions by reviewing account history and customizing dashboards with relevant metrics (e.g., patient enrollment rates).
- Conduct quarterly satisfaction surveys and hold debrief sessions post-milestones.
- Integrate feedback into process improvements for continuous enhancement.
By fostering stronger relationships through consistent and personalized communication, you boost retention and drive trial success. 🤝
Mistake 3: Insufficient Resource Allocation and Support
Insufficient resource allocation is a critical mistake in clinical research key account management. ⚠️ Understaffing or lacking specialized expertise leads to delayed responses, missed opportunities, and strained client relationships. For example, assigning a general account manager without clinical trial experience to a complex oncology study can result in misunderstandings of protocol requirements and compliance issues.
Practical steps to avoid this:
- Assess account complexity and allocate staff with relevant expertise (e.g., therapeutic area knowledge).
- Implement ongoing training on clinical research processes, GCP guidelines, and risk mitigation.
- Develop a crisis management protocol with clear roles, communication plans, and escalation paths.
- Use checklists for regular account reviews to ensure resource adequacy and support readiness.
Checklist for resource readiness:
- [ ] Staff assigned based on expertise and account needs
- [ ] Regular training sessions scheduled
- [ ] Crisis plan documented and tested
- [ ] Account reviews conducted quarterly
By investing in the right people and proactive strategies, you enhance account satisfaction and trial success. 🛠️
Mistake 4: Ignoring Competitor Actions and Market Trends
Ignoring competitor actions and market trends is a critical mistake in clinical research account management. 🌐 When you fail to monitor competitors' strategies—such as new service offerings, pricing changes, or partnership announcements—you risk losing key accounts to more agile rivals. Similarly, overlooking industry shifts like regulatory updates or emerging technologies can leave your services outdated and less competitive.
Leveraging tools like RivalSense can provide real-time insights to stay ahead. Here are examples of valuable competitor intelligence:
-
Event Participation Insight: Kristjan Lepik, CEO of Arbonics, will share a big company milestone on stage at Slush in Helsinki.
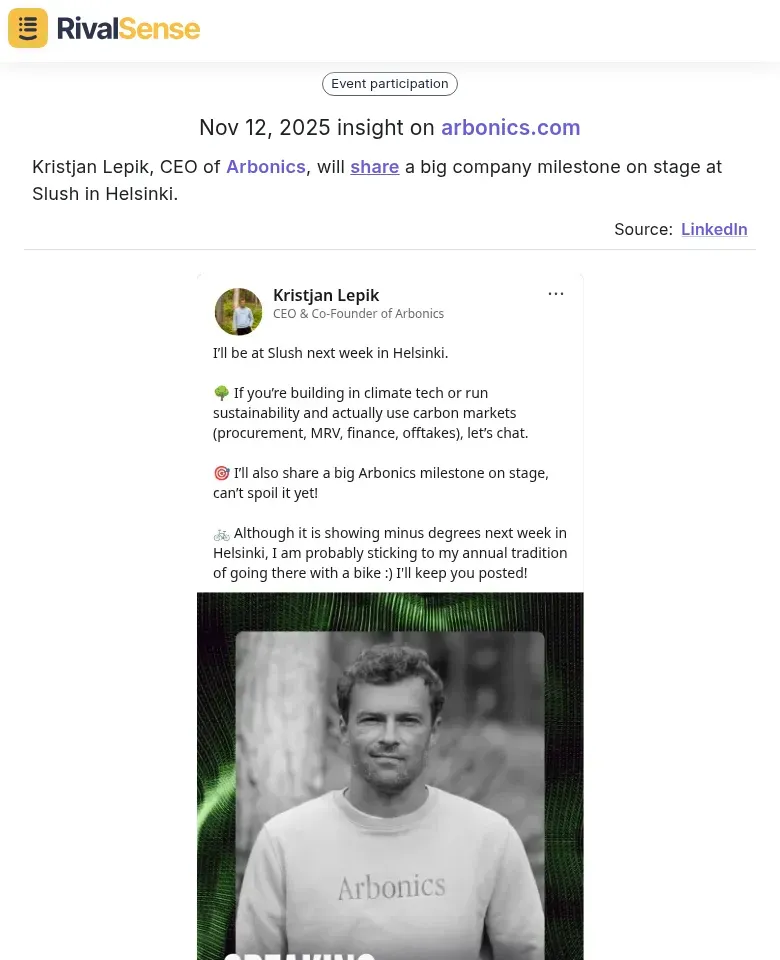 This type of insight is valuable because it alerts you to competitor announcements and industry events, helping you anticipate market moves and align your strategies.
This type of insight is valuable because it alerts you to competitor announcements and industry events, helping you anticipate market moves and align your strategies. -
Management Change Insight: Hugh Walker joined Labrys as Business Director in the United Kingdom, previously unspecified.
 Tracking management changes helps you understand competitor's strategic hires and potential shifts in business direction, enabling you to prepare competitive responses.
Tracking management changes helps you understand competitor's strategic hires and potential shifts in business direction, enabling you to prepare competitive responses. -
Role Change Insight: Derek Murphy joined Vanta as Director - Account Management EMEA in Ireland, previously Sales Director at Okta.
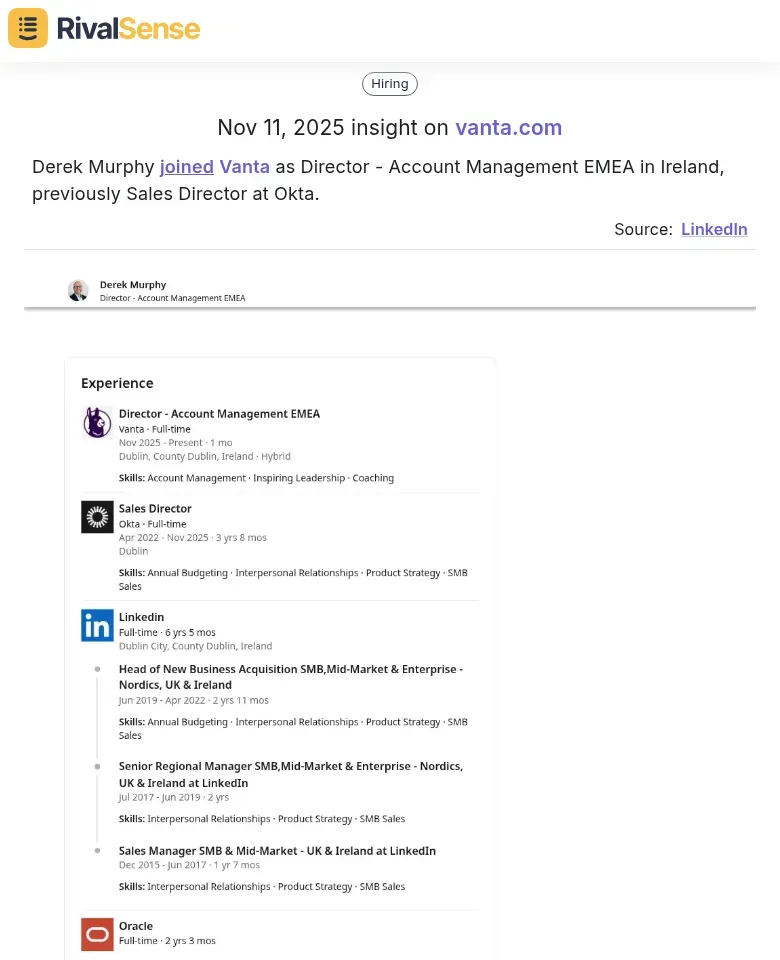 This insight is crucial for identifying talent movements and competitive threats in key regions, informing your hiring and expansion plans.
This insight is crucial for identifying talent movements and competitive threats in key regions, informing your hiring and expansion plans.
Practical steps to avoid this mistake:
- Conduct regular competitor audits using tools like RivalSense to track websites, clinical trial registries, and industry reports.
- Set up alerts for competitor news, patent filings, and regulatory submissions.
- Analyze trends by attending conferences and engaging with key opinion leaders.
- Act on insights by adjusting your service portfolio, pricing, or communication strategies.
By staying vigilant, you can proactively address threats and seize advantages in a dynamic market. 🚀
Best Practices and Conclusion
To avoid key account management mistakes in clinical research, implement structured quarterly account reviews with clear KPIs. 📊 Track study milestones, budget adherence, and sponsor satisfaction scores to ensure alignment and continuous improvement. Foster long-term partnerships by offering value-added services like regulatory guidance or risk mitigation workshops, which deepen trust and demonstrate commitment.
Key takeaways for success:
- Prioritize proactive communication and goal alignment with sponsors.
- Avoid siloed operations by training teams on collaborative problem-solving.
- Leverage competitor tracking tools to benchmark performance and adapt to market changes.
Implementation checklist:
- [ ] Define account-specific KPIs
- [ ] Schedule regular strategy sessions
- [ ] Solicit feedback for iterative improvements
This approach ensures sustained growth and minimizes errors in high-stakes clinical trials. 💡 Ready to enhance your competitor intelligence? Try out RivalSense for free at https://rivalsense.co/ to assist with the challenges described in this post. Get your first competitor report today and start making data-driven decisions!
📚 Read more
👉 How Competitors Countered Lightdash's Strategic Partnership
👉 Facebook Competitor Insights Framework: Templates for Key Account Tracking
👉 How to Outsmart Vacation Rental Software Competitors with Feature Analysis
👉 Unlocking Growth Through Competitive Partnership Intelligence
👉 Leveraging Competitor Event Participation for Strategic Insights
